what is the ability of a substance to resist burning called
A photoresist (besides known just as a resist) is a light-sensitive fabric used in several processes, such as photolithography and photoengraving, to grade a patterned blanket on a surface. This process is crucial in the electronic industry.[ane]
The procedure begins by coating a substrate with a calorie-free-sensitive organic material. A patterned mask is then practical to the surface to block light, and then that only unmasked regions of the cloth will be exposed to light. A solvent, called a developer, is then applied to the surface. In the case of a positive photoresist, the photo-sensitive cloth is degraded past light and the developer will deliquesce away the regions that were exposed to light, leaving behind a blanket where the mask was placed. In the instance of a negative photoresist, the photosensitive material is strengthened (either polymerized or cross-linked) by light, and the programmer will dissolve away but the regions that were not exposed to light, leaving behind a blanket in areas where the mask was not placed.
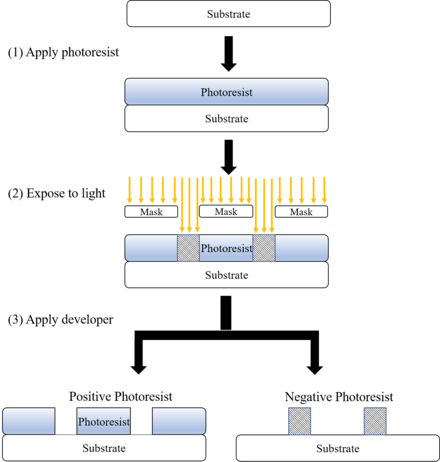
Photoresist of Photolithography
A BARC coating (Bottom Anti-Reflectant Blanket) may be applied earlier the photoresist is applied, to avert reflections from occurring under the photoresist and to better the photoresist's performance at smaller semiconductor nodes.[two] [3] [4]
Definitions [edit]
Positive photoresist [edit]
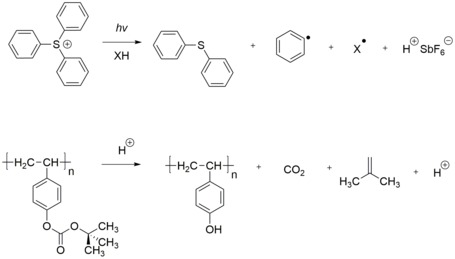
A positive photoresist case, whose solubility would change by the photogenerated acid. The acid deprotects the tert-butoxycarbonyl (t-BOC), inducing the resist from alkali insoluble to alkali soluble. This was the beginning chemically amplified resist used in the semiconductor industry, which was invented by Ito, Willson, and Frechet in 1982.[5]

An example for unmarried component positive photoresist
A positive photoresist is a type of photoresist in which the portion of the photoresist that is exposed to low-cal becomes soluble to the photoresist developer. The unexposed portion of the photoresist remains insoluble to the photoresist developer.
Negative photoresist [edit]
A negative photoresist is a type of photoresist in which the portion of the photoresist that is exposed to low-cal becomes insoluble to the photoresist developer. The unexposed portion of the photoresist is dissolved by the photoresist developer.

A crosslinking of a polyisoprene rubber by a photoreactive biazide as negative photoresist
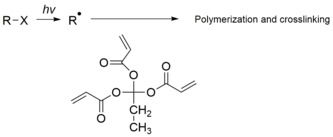
A radical induced polymerization and crosslinking of an acrylate monomer as negative photoresist
Differences between positive and negative resist [edit]
The following table[6] is based on generalizations which are generally accepted in the microelectromechanical systems (MEMS) fabrication industry.
| Characteristic | Positive | Negative |
| Adhesion to silicon | Fair | Excellent |
| Relative cost | More expensive | Less expensive |
| Developer base | Aqueous | Organic |
| Solubility in the programmer | Exposed region is soluble | Exposed region is insoluble |
| Minimum feature | 0.5 µm | 2 µm |
| Footstep coverage | Better | Lower |
| Wet chemical resistance | Off-white | Fantabulous |
Types [edit]
Based on the chemical structure of photoresists, they tin be classified into 3 types: photopolymeric, photodecomposing, photocrosslinking photoresist.
Photopolymeric photoresist is a type of photoresist, usually allyl monomer, which could generate free radical when exposed to calorie-free, then initiates the photopolymerization of monomer to produce a polymer. Photopolymeric photoresists are usually used for negative photoresist, eastward.1000. methyl methacrylate.
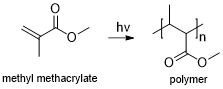
Photopolymerization of methyl methacrylate monomers under UV that resulting into polymer
Photodecomposing photoresist is a blazon of photoresist that generates hydrophilic products under light. Photodecomposing photoresists are unremarkably used for positive photoresist. A typical instance is azide quinone, east.g. diazonaphthaquinone (DQ).

Photolysis of a dizaonaphthoquinone that leads to a much more polar surround, which allows aqueous base to dissolve a Bakelite-type polymer.
Photocrosslinking photoresist is a type of photoresist, which could crosslink concatenation by chain when exposed to light, to generate an insoluble network. Photocrosslinking photoresist are usually used for negative photoresist.
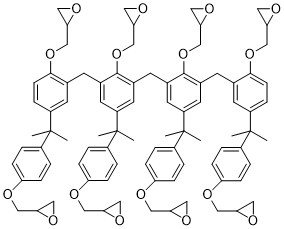
Chemical construction of SU-8 (a single molecule contains 8 epoxy groups)
Mechanism of SU-eight for negative photoresist
Off-Stoichiometry Thiol-Enes (OSTE) polymers[vii]
For self-assembled monolayer SAM photoresist, first a SAM is formed on the substrate by self-assembly. Then, this surface covered by SAM is irradiated through a mask, similar to other photoresist, which generates a photo-patterned sample in the irradiated areas. And finally programmer is used to remove the designed part (could be used as both positive or negative photoresist).[8]
Light sources [edit]
Absorption at UV and shorter wavelengths [edit]
In lithography, decreasing the wavelength of light source is the most efficient way to accomplish higher resolution.[ix] Photoresists are about unremarkably used at wavelengths in the ultraviolet spectrum or shorter (<400 nm). For example, diazonaphthoquinone (DNQ) absorbs strongly from approximately 300 nm to 450 nm. The absorption bands tin be assigned to n-π* (S0–S1) and π-π* (S1–S2) transitions in the DNQ molecule.[ citation needed ] In the deep ultraviolet (DUV) spectrum, the π-π* electronic transition in benzene[10] or carbon double-bond chromophores appears at around 200 nm.[ commendation needed ] Due to the appearance of more than possible absorption transitions involving larger energy differences, the absorption tends to increase with shorter wavelength, or larger photon energy. Photons with energies exceeding the ionization potential of the photoresist (tin can be equally depression equally 5 eV in condensed solutions)[11] can also release electrons which are capable of boosted exposure of the photoresist. From about 5 eV to nearly xx eV, photoionization of outer "valence band" electrons is the main absorption machinery.[12] In a higher place 20 eV, inner electron ionization and Auger transitions get more of import. Photon absorption begins to subtract as the X-ray region is approached, every bit fewer Auger transitions between deep atomic levels are allowed for the higher photon energy. The absorbed free energy tin can drive further reactions and ultimately dissipates as heat. This is associated with the outgassing and contagion from the photoresist.
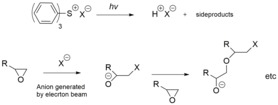
Electron-axle exposure [edit]
Photoresists tin can also be exposed by electron beams, producing the same results equally exposure past light. The main difference is that while photons are absorbed, depositing all their energy at once, electrons deposit their energy gradually, and scatter within the photoresist during this procedure. As with high-energy wavelengths, many transitions are excited by electron beams, and heating and outgassing are all the same a concern. The dissociation energy for a C-C bond is 3.6 eV. Secondary electrons generated past principal ionizing radiation take energies sufficient to dissociate this bond, causing scission. In add-on, the low-energy electrons accept a longer photoresist interaction time due to their lower speed; essentially the electron has to be at rest with respect to the molecule in lodge to react almost strongly via dissociative electron attachment, where the electron comes to rest at the molecule, depositing all its kinetic energy.[13] The resulting scission breaks the original polymer into segments of lower molecular weight, which are more readily dissolved in a solvent, or else releases other chemical species (acids) which catalyze further scission reactions (see the discussion on chemically amplified resists below). It is not common to select photoresists for electron-beam exposure. Electron beam lithography commonly relies on resists defended specifically to electron-axle exposure.
Parameters [edit]
Physical, chemical and optical properties of photoresists influence their selection for unlike processes.[14]
- Resolution is the power to differ the neighboring features on the substrate. Critical dimension (CD) is a main measure of resolution.
The smaller the critical dimension is, the higher resolution would exist.
- Contrast is the divergence from exposed portion to unexposed portion. The college the contrast is, the more than obvious the difference between exposed and unexposed portions would exist.
- Sensitivity is the minimum free energy that is required to generate a well-defined feature in the photoresist on the substrate, measured in mJ/cmtwo. The sensitivity of a photoresist is important when using deep ultraviolet (DUV) or extreme-ultraviolet (EUV).
- Viscosity is a mensurate of the internal friction of a fluid, affecting how easily it will flow. When it is needed to produce a thicker layer, a photoresist with higher viscosity will be preferred.
- Adherence is the adhesive strength betwixt photoresist and substrate. If the resist comes off the substrate, some features will be missing or damaged.
- Anti-etching is the power of a photoresist to resist the high temperature, dissimilar pH environment or the ion bombardment in the process of post-modification.
- Surface tension is the tension that induced by a liquid tended to minimize its area, which is caused by the allure of the particles in the surface layer. In order to better moisture the surface of substrate, photoresists are required to possess relatively low surface tension.
Positive photoresist [edit]
DNQ-Novolac photoresist [edit]
One very common positive photoresist used with the I, G and H-lines from a mercury-vapor lamp is based on a mixture of diazonaphthoquinone (DNQ) and novolac resin (a phenol formaldehyde resin). DNQ inhibits the dissolution of the novolac resin, simply upon exposure to low-cal, the dissolution rate increases fifty-fifty beyond that of pure novolac. The machinery by which unexposed DNQ inhibits novolac dissolution is non well understood, but is believed to be related to hydrogen bonding (or more exactly diazocoupling in the unexposed region). DNQ-novolac resists are developed past dissolution in a basic solution (usually 0.26N tetramethylammonium hydroxide (TMAH) in water).
Negative photoresist [edit]
Epoxy-based polymer [edit]
I very common negative photoresist is based on epoxy-based polymer. The mutual product name is SU-8 photoresist, and it was originally invented by IBM, but is now sold by Microchem and Gersteltec. One unique property of SU-viii is that it is very difficult to strip. Every bit such, it is frequently used in applications where a permanent resist pattern (one that is non strippable, and tin can even exist used in harsh temperature and force per unit area environments) is needed for a device.[15] Mechanism of epoxy-based polymer is shown in i.ii.3 SU-eight.
Off-stoichiometry thiol-enes(OSTE) polymer [edit]
In 2016, OSTE Polymers were shown to possess a unique photolitography mechanism, based on diffusion-induced monomer depletion, which enables high photostructuring accuracy. The OSTE polymer textile was originally invented at the KTH Royal Institute of Engineering science, but is now sold by Mercene Labs. Whereas the material has backdrop similar to those of SU8, OSTE has the specific reward that information technology contains reactive surface molecules, which make this material bonny for microfluidic or biomedical applications.[14]
Hydrogen Silsesquioxane (HSQ) [edit]
HSQ is a common negative resist for e-axle, just also useful for photolithography. Originally invented past Dow Corning (1970),[16] and now produced (2017) by Applied Quantum Materials Inc. (AQM). Unlike other negative resists, HSQ is inorganic and metallic-gratis. Therefore, exposed HSQ provides a depression dielectric constant (depression-k) Si rich oxide. A comparative study against other photoresists was reported in 2015 (Dow Corning HSQ).[17]
Applications [edit]
Microcontact printing [edit]
Microcontact printing was described by Whitesides Grouping in 1993. Generally, in this techniques, an elastomeric stamp is used to generate ii-dimensional patterns, through press the "ink" molecules onto the surface of a solid substrate.[18]


rightInking and contact process
Step i for microcontact press. A scheme for the creation of a polydimethylsiloxane (PDMS) chief stamp. Pace ii for microcontact printing A scheme of the inking and contact process of microprinting lithography.
Printed circuit boards [edit]
The industry of printed circuit boards is one of the most important uses of photoresist. Photolithography allows the complex wiring of an electronic system to be rapidly, economically, and accurately reproduced as if run off a printing press. The full general process is applying photoresist, exposing image to ultraviolet rays, and so etching to remove the copper-clad substrate.[19]

A printed circuit lath-4276
Patterning and carving of substrates [edit]
This includes specialty photonics materials, MicroElectro-Mechanical Systems (MEMS), drinking glass printed circuit boards, and other micropatterning tasks. Photoresist tends not to be etched by solutions with a pH greater than 3.[20]

A micro-electrical-mechanical cantilever inproduced past photoetching
Microelectronics [edit]
This awarding, mainly applied to silicon wafers/silicon integrated circuits is the most adult of the technologies and the virtually specialized in the field.[21]
![]()
See too [edit]
- Photopolymer
- Hardmask
References [edit]
- ^ Eric, Anslyn; Dougherty, Dennis. Modern physical organic chemistry. University Science Books.
- ^ "Top Anti-reflective Coatings vs Bottom Anti-reflective Coatings".
- ^ MicroChemicals. "Basics of Microstructuring: Anti-Reflective Coatings" (PDF). Microchemicals GmbH. Retrieved 2020-01-31 .
- ^ "AR™ 10L Bottom Anti-Reflectant Coating (BARC) | DuPont". www.dupont.com.
- ^ Ito, H.; Willson, C. Thou.; Frechet, J. H. J. (1982-09-01). "New UV Resists with Negative or Positive Tone". 1982 Symposium on VLSI Applied science. Assimilate of Technical Papers: 86–87.
- ^ Madou, Marc (2002-03-13). Fundamentals of Microfabrication. CRC Press. ISBN978-0-8493-0826-0.
- ^ Saharil, Farizah; Forsberg, Fredrik; Liu, Yitong; Bettotti, Paolo; Kumar, Neeraj; Niklaus, Frank; Haraldsson, Tommy; Van Der Wijngaart, Wouter; Gylfason, Kristinn B (2013). "Dry adhesive bonding of nanoporous inorganic membranes to microfluidic devices using the OSTE(+) dual-cure polymer". Journal of Micromechanics and Microengineering. 23 (2): 025021. Bibcode:2013JMiMi..23b5021S. doi:ten.1088/0960-1317/23/2/025021.
- ^ Huang, Jingyu; Dahlgren, David A.; Hemminger, John C. (1994-03-01). "Photopatterning of Self-Assembled Alkanethiolate Monolayers on Gold: A Simple Monolayer Photoresist Utilizing Aqueous Chemical science". Langmuir. 10 (3): 626–628. doi:10.1021/la00015a005. ISSN 0743-7463.
- ^ Bratton, Daniel; Yang, Da; Dai, Junyan; Ober, Christopher K. (2006-02-01). "Recent progress in high resolution lithography". Polymers for Advanced Technologies. 17 (2): 94–103. doi:10.1002/pat.662. ISSN 1099-1581. S2CID 55877239.
- ^ Ishii, Hiroyuki; Usui, Shinji; Douki, Katsuji; Kajita, Toru; Chawanya, Hitoshi; Shimokawa, Tsutomu (2000-01-01). Houlihan, Francis M (ed.). "Pattern and lithographic performances of 193-specific photoacid generators". Advances in Resist Engineering science and Processing Xvii. 3999: 1120–1127. Bibcode:2000SPIE.3999.1120I. doi:ten.1117/12.388276. S2CID 98281255.
- ^ Belbruno, Joseph (1990). "Multiphoton-induced chemistry of phenol in hexane at 266 nm". Chemical Physics Letters. 166 (2): 167–172. Bibcode:1990CPL...166..167B. doi:10.1016/0009-2614(ninety)87271-r.
- ^ Weingartner, Joseph C; Draine, B. T; Barr, David K (2006). "Photoelectric Emission from Dust Grains Exposed to Extreme Ultraviolet and X-Ray Radiation". The Astrophysical Journal. 645 (2): 1188–1197. arXiv:astro-ph/0601296. Bibcode:2006ApJ...645.1188W. doi:10.1086/504420. S2CID 13859981.
- ^ Braun, M; Gruber, F; Ruf, Grand. -West; Kumar, South. V. K; Illenberger, Due east; Hotop, H (2006). "IR photon enhanced dissociative electron attachment to SF6: Dependence on photon, vibrational, and electron energ". Chemical Physics. 329 (1–3): 148. Bibcode:2006CP....329..148B. doi:x.1016/j.chemphys.2006.07.005.
- ^ a b Greener, Jesse; Li, Wei; Ren, Judy; Voicu, Dan; Pakharenko, Viktoriya; Tang, Tian; Kumacheva, Eugenia (2010-02-02). "Rapid, price-efficient fabrication of microfluidic reactors in thermoplastic polymers by combining photolithography and hot embossing". Lab Chip. 10 (iv): 522–524. doi:10.1039/b918834g. ISSN 1473-0189. PMID 20126695. S2CID 24567881.
- ^ DeForest, William S (1975). Photoresist: materials and processes. McGraw-Hill Companies.
- ^ Frye, Cecil L.; Collins, Ward T. (1970-09-01). "Oligomeric silsesquioxanes, (HSiO3/2)n". Journal of the American Chemical Society. 92 (xix): 5586–5588. doi:10.1021/ja00722a009. ISSN 0002-7863.
- ^ Mojarad, Nassir; Gobrecht, Jens; Ekinci, Yasin (2015-03-18). "Beyond EUV lithography: a comparative study of efficient photoresists' functioning". Scientific Reports. five (1): 9235. doi:10.1038/srep09235. ISSN 2045-2322.
- ^ "Self-assembled Monolayer Films: Microcontact Printing" (PDF).
- ^ Montrose, Marking I (1999). The Electronic Packaging Handbook. CRC Printing.
- ^ Novak, R.Due east (2000). Cleaning Technology in Semiconductor Device Manufacturing. Electrochemical Lodge Inc. ISBN978-1566772594.
- ^ Silicon photonics. Springer Science & Business Media. 2004.
Source: https://en.wikipedia.org/wiki/Photoresist
0 Response to "what is the ability of a substance to resist burning called"
Post a Comment